Quality system improvement implementation
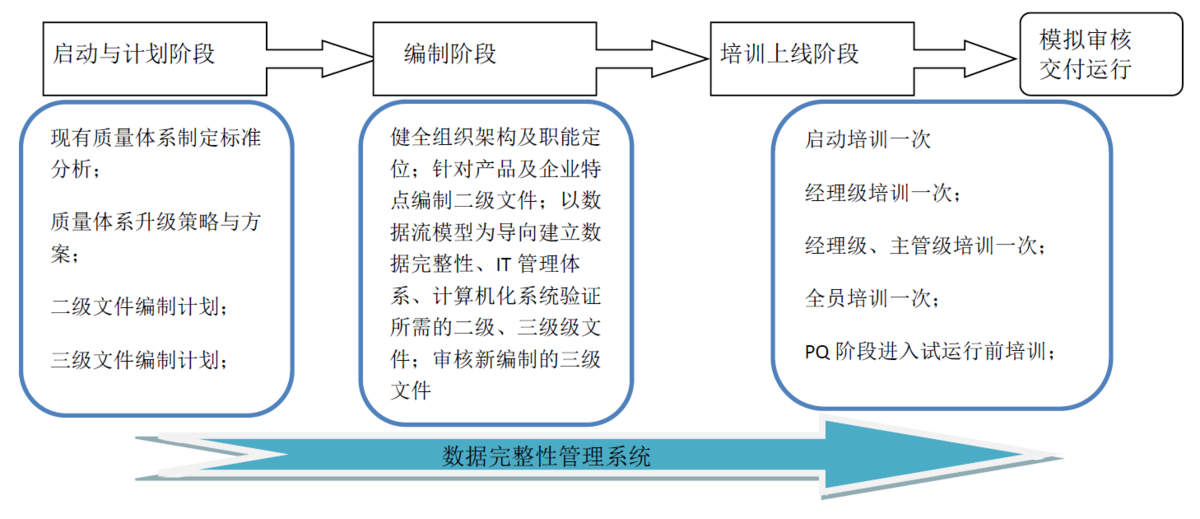
Main work steps:
Analyze the corporate culture and project objectives with the owner team and determine the strategy for entering a new quality system
Identify the design strategy of the process with the design team
Train key personnel in the quality team based on the characteristics of cGMP and FDA
Formulate the basic framework of the quality system based on its own characteristics and process characteristics, self-control level, etc.
Expert review quality management system framework
List the main procedures and key documents
Assist the owner to complete the detailed process documentation and management documentation
Review of process files and management files
Employee training
After completing the performance verification (PQ), organize a mock audit.
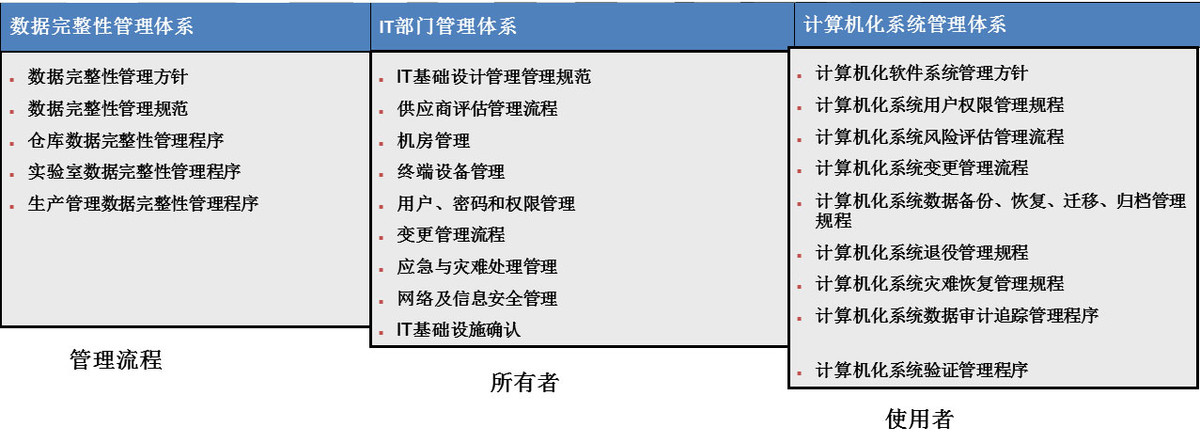
Provide customers with a scientific data integrity system to build a program: according to the internal owners, users to divide functions, according to the data life cycle (data creation, data processing, data reporting and use, data retention and retrieval, data destruction and regeneration) management.